Leila Sullivan, Zeynep Celik, and Amy Killelea
Prior authorization (PA) is a utilization management technique used by health insurers that requires providers to seek approval from the insurance plan before the plan will agree to pay for a covered procedure, service, or medication. Insurers can leverage PA to control health care spending, both by negotiating lower prices for services that they do not subject to PA and using PA to limit access to certain higher-price services.
While insurers contend that PA also allows them to ensure care is clinically appropriate, PA has been linked to adverse health outcomes for patients, as well as delays and frustration in the US health care system. The administrative costs associated with PA are also significant, and a profitable cottage industry of middlemen has grown to help both insurers and providers navigate PA.
In response to growing concerns about PA abuses, policy makers at the federal and state levels are increasingly implementing reforms that target the processes insurers use, the substance of their requirements, or both. This article explores key trends in PA reform and what those reforms mean for consumers. We primarily focus on the commercial health insurance market, although some policies apply more broadly.
Federal-Level Action
Under the Biden administration, the Centers for Medicare and Medicaid Services (CMS) finalized a new rule on the use of PA in federal Marketplace plans as well as those offered through Medicare Advantage and Medicaid/CHIP. Most significantly, the rules require that by 2027 insurers must have in place an application programming interface through which consumers and providers can learn:
- whether PA applies to specific items or services,
- any associated documentation requirements, and
- the status of pending or processed requests, including the specific reasons for any denials.
Additionally, starting in 2026, affected payers must post online specified data on PA requests and determinations. The rules also standardize certain aspects of insurer PA processes beginning in 2026. For example, insurers must put timelines in place for PA determinations for Medicare Advantage and Medicaid/CHIP such that standard requests are addressed within seven calendar days and, for expedited requests, within 72 hours. For federal Marketplace plans, preexisting timelines remain in place, which allow plans up to 15 days to process standard requests, while expedited requests are limited to 72 hours.
Across all payers and provisions, the above rules apply only to medical benefits and not prescription drugs, leaving a large regulatory gap for patients and providers. In a separate rule finalized this summer, however, CMS has required that certified electronic health record systems allow for electronic prior authorization of prescription drugs.
Then in June 2025, health insurance industry leaders in coordination with the Trump administration, voluntarily pledged to streamline and improve their PA processes across markets. Beginning in 2026, pledge participants promise to reduce the volume of services subject to PA, honor PA approvals by other plans during coverage transitions, and provide clear explanations of their PA determinations. By the following year, they commit to making real-time approvals on most requests and standardizing electronic PA request submissions.
State Action
Many states are taking matters into their own hand, adopting their own rules for the fully insured health insurance market. During the 2024 legislative session, 10 states passed legislation reforming PA. Since January 1, 2025, at least 18 states have taken legislative action on PA (see exhibit 1 here).
Exhibit 1: 2025 Enacted Legislation On Prior Authorization
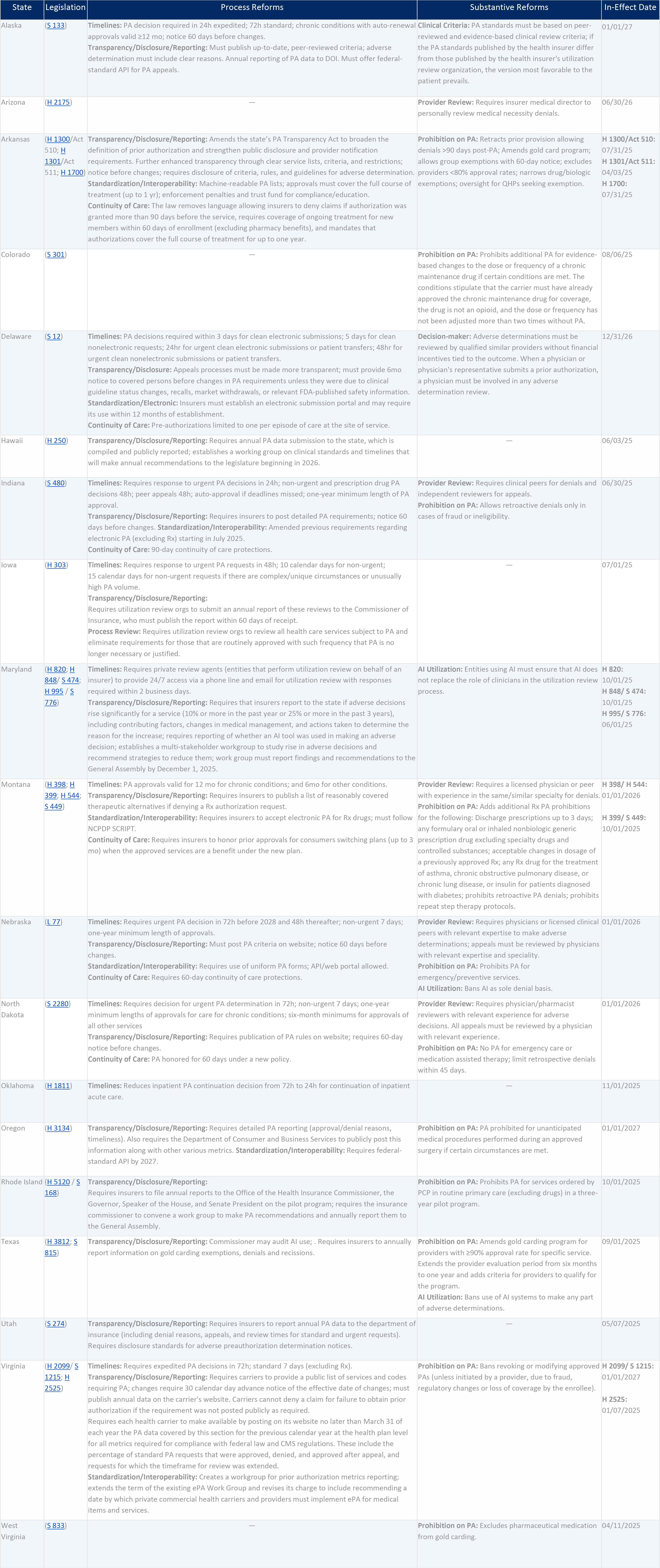
Source: The authors’ research includes state level actions enacted between January 1st, 2025 and August 30th 2025
State reforms generally fall along two dimensions: process changes and substantive changes.
Like the federal interoperability rule, many state-driven changes address procedural aspects of PA such as:
- how quickly insurers must process requests,
- how long approvals last,
- which channels can be used for PA requests, and
- what information insurers must disclose to consumers and providers.
Other state reforms are more substantive. Some aim to prevent insurers from denying access to clinically recommended care and treatment. Others seek to ensure that only health care professionals with relevant expertise can make denial decisions.
Process Reforms
A primary focus of recent state procedural PA reforms has been timelines. These can encompass both the required turnaround times for approving or denying a PA request and the duration for which an approval remains valid. New laws in Alaska, Delaware, Indiana, Iowa, Nebraska, North Dakota, Oklahoma, and Virginia require insurers to process urgent prior authorization requests on timelines that range from 24 to 72 hours for urgent requests, and 2 to 15 days for non-urgent requests, depending on the state.
Indiana’s law adds a strong incentive for timely insurer PA processing: If the insurer misses the deadline, the request is automatically approved. Alaska and Montana defined how long an approval lasts for conditions requiring chronic care, setting a minimum duration of 12 months. Alaska’s law also requires that if the treatment plan for a chronic condition remains the same after the initial 12-month period, the PA is automatically renewed.
Several states (Arkansas, Indiana, Montana, North Dakota, and Nebraska) have also taken measures to ensure continuity of care for patients and maintain PA approvals for the first two to three months following a change in health insurance coverage. These measures aim to reduce delays in care, minimize repeated paperwork, and provide patients, especially those with ongoing health needs, with greater stability and continuity in their treatment.
As mentioned above, CMS issued a final rule in early 2024 to improve PA processes in Medicare Advantage, Medicaid/CHIP, and plans offered on the federal Marketplace. A core focus of the rule was to require plans to make information on PA and plan approval criteria easily (and electronically) available to patients and providers. State lawmakers have also taken action to require plans to be more transparent about their PA processes, with several states in 2025 enacting measures to increase patient and provider access to PA information and to require plans to report on PA to state regulators.
Nebraska, Arkansas, and North Dakota, for example, now require insurers to publicly post PA policies, clinical criteria, and documentation requirements in a clear and accessible format for patients. All states in exhibit 1, except Indiana, also include prescription drug PA in their laws in an effort to fill the major gap in the federal rule, which excluded prescription drugs from the PA reforms included in the rule. In addition to transparency, these reforms aim to standardize electronic PA processes in ways that enable faster, more consistent decision making and reduce administrative burdens for providers and patients. By making this information readily available, patients can better understand what is required for approval, anticipate potential delays, and make more informed decisions about their care.
Substantive Reforms
In 2025, several states enacted substantive reforms to PA processes, focusing on how clinical review criteria are defined, who makes determinations, and whether certain services or providers can be excluded from PA.
Alaska and Nebraska now require carriers to rely on peer-reviewed and evidence-based clinical review criteria and to ensure that adverse determinations are made by qualified clinical peers. These reforms aim to improve adherence to best practices. Montana, North Dakota, and Virginia strengthened patient protections by prohibiting retroactive PA denials, with North Dakota and Virginia allowing limited exceptions.
States have also strengthened oversight of decision making in PA determinations. Arizona now requires denials based on medical necessity to be individually reviewed by a medical director. Several states addressed the growing use of artificial intelligence (AI) in utilization management amid concerns linking it to higher denials. Maryland’s reform requires carriers to disclose the use of AI in reporting, reinforces that human oversight is mandatory, and prohibits AI tools from being used to deny, modify, or delay care. Texas similarly bans automated systems from issuing adverse determinations without human review, although it does permit AI for administrative support or fraud detection. These provisions aim to ensure technology is supporting clinical judgment instead of replacing it.
Other reforms have targeted PA requirements by exempting certain low-risk services and high-performing providers, while streamlining approval processes or routine or ongoing treatments.
Some new laws have allowed for expanded “gold carding” programs under which insurers exempt certain providers from prior authorization requirements based on their track record of approvals or on other standards. Arkansas amended its gold carding program to refine evaluation periods and eligibility criteria. And Texas now waives PA for providers with a 90 percent approval rate for a service. Rhode Island eliminated PA for routine primary care services ordered by a primary care physician during a three-year pilot program beginning October 1, 2025, while Colorado removed additional PA requirements for certain dose and frequency adjustments to previously approved chronic maintenance drugs. Montana prohibits PA for both short- and long-acting insulin (whether generic or brand name) and limits repeat step-therapy protocols when a patient has already failed required drugs. Together, these changes reflect a broader state-level push to reduce delays in care while preserving oversight where clinically appropriate.
Looking Ahead
The growing number of reforms reflects an increased recognition that, under the current health care system, PA often delays care and creates significant administrative burdens. Process reforms designed to streamline procedures and shorten approval timelines, along with substantive reforms intended to ensure more evidence-based determinations, represent meaningful progress. Many states’ 2025 legislative actions complement federal action. Nonetheless, with most ambitious reforms happening at the state level, patients’ experiences will continue to vary widely based on where they live and the type of coverage they hold.
Authors’ Note
The authors would like to thank Billy Derring and Vrudhi Raimugia for their support on this article.
Leila Sullivan, Zeynep Celik, and Amy Killelea “Prior Authorization Reform Heats Up” November 24, 2025, https://www.healthaffairs.org/content/forefront/prior-authorization-reform-heats-up. Copyright © 2025 Health Affairs by Project HOPE – The People-to-People Health Foundation, Inc.